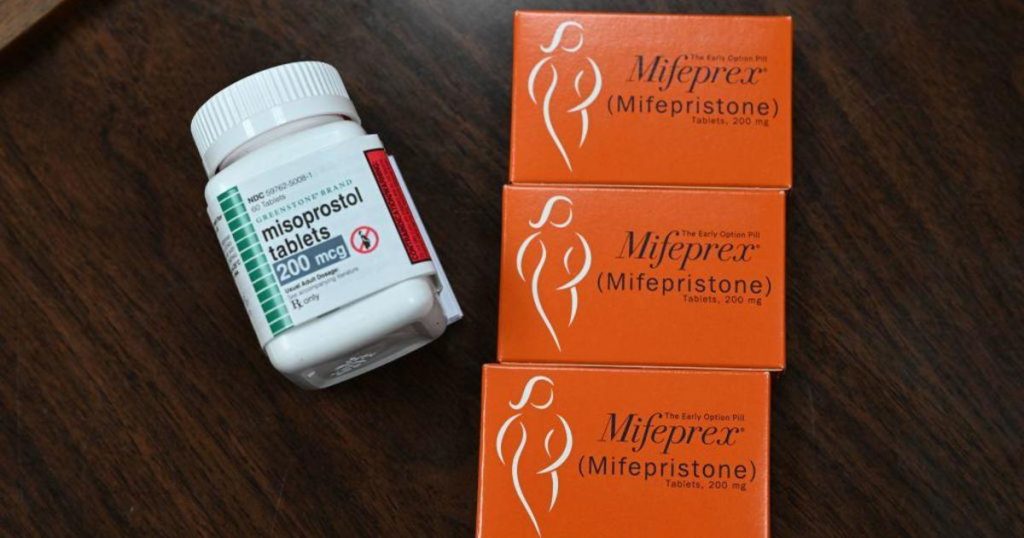
The Supreme Court is set to hear arguments in a case involving the accessibility of the abortion pill, mifepristone. The FDA has taken steps in recent years to make the medication easier to obtain, including allowing it to be taken up to 10 weeks into pregnancy and delivered through the mail. Anti-abortion groups have challenged these changes, claiming the FDA violated the law. A ruling against the FDA could restrict access to mifepristone nationwide, impacting states with laws protecting abortion rights.
The case comes after the Supreme Court’s decision in 2022 to unwind the constitutional right to abortion, returning the issue to the states. Another case involves whether federal law requires emergency room doctors in states that ban abortion to perform the procedure on pregnant patients in life-threatening situations. The court’s review of the mifepristone case also follows findings that medication abortions in the U.S. have increased since the overturning of Roe v. Wade, with medication abortions accounting for a growing percentage of all abortions.
The challenge to the FDA’s changes surrounding mifepristone was filed by a group of medical associations that oppose abortion rights. The FDA’s approval of mifepristone in 2000 and subsequent changes in 2016 and 2021 have come under legal scrutiny. The federal judge overseeing the case ruled that the FDA’s actions were likely unlawful, prompting appeals that ultimately led to the Supreme Court preserving access to mifepristone pending a final decision. The Justice Department and the maker of mifeprex have asked the Supreme Court to reverse the lower court’s decision.
Arguments in the case center around whether the FDA had the authority to make changes to mifepristone’s usage and whether those changes were based on a sound medical justification. The Biden administration has argued that the doctors challenging the changes do not have standing to sue, while lawyers for the medical groups claim the FDA’s actions have increased health and safety risks for women seeking abortions. Pharmaceutical companies and former heads of the FDA have warned that upholding the lower court’s decision could undermine the drug approval process and hinder patient access to critical medications.
The outcome of the case could impact access to medication abortions in the U.S. and set a precedent for future legal challenges to FDA drug approvals. The Supreme Court’s decision is expected by the end of June, and the implications could be far-reaching for women’s reproductive health and access to abortion care. The case highlights the ongoing battle over abortion rights in the U.S. and the complex legal and medical issues at stake.