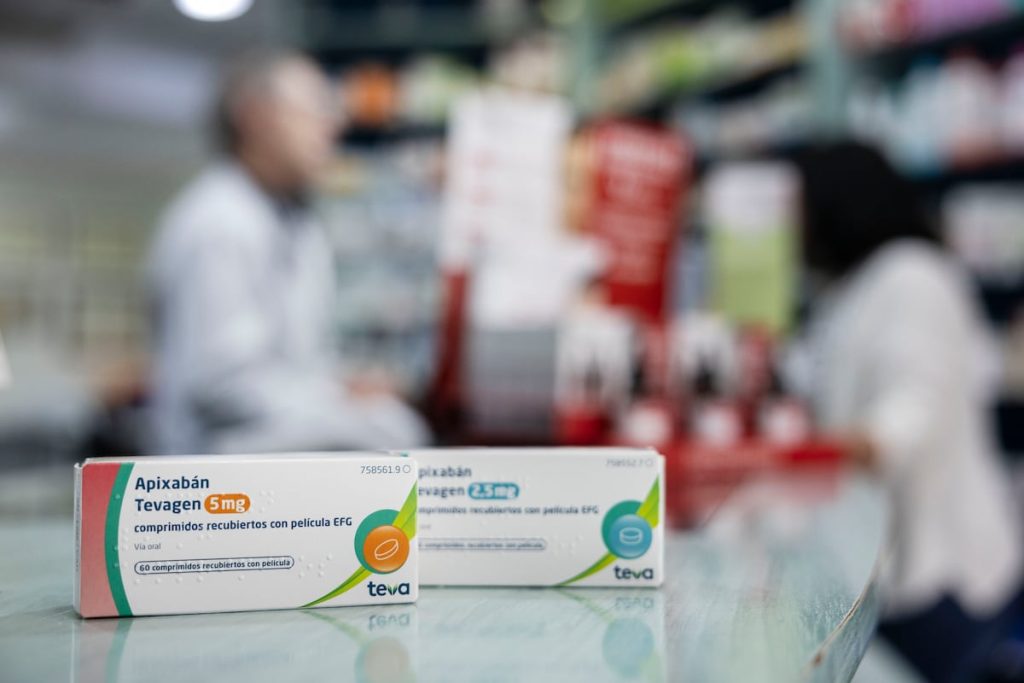
The Supreme Court has given the green light for the pharmaceutical company Teva to market the generic version of the anticoagulant drug apixaban in Spain, which was previously only sold by Bristol Myers Squibb (BMS) under the brand name Eliquis. This decision lifts the precautionary measures decreed two and a half months ago by a court in Madrid that halted the arrival of the generic to pharmacies and ends a legal dispute that was costing the public health system 380,000 euros daily. This has prevented autonomous communities, which bear the pharmaceutical costs, from saving 23.2 million euros since March 1, the initial date set for the release of the generic. The introduction of the generic apixaban is expected to save the public health system nearly 138 million euros annually, as the price of the drug will drop from the current 81.96 euros (the cost of a box of Eliquis) to 45.08 euros, a 45% reduction. The usual consumption per patient is one box per month.
In total, over 310,000 patients in Spain take the drug for indications covered by the public health system. The most common indication is non-valvular atrial fibrillation, which affects between 15% and 20% of the population over 80 years old. As a treatment for chronic patients, who only pay a maximum of 10% of the drug price with a cap of 4.2 euros per month, this will remain the amount patients pay for the drug. However, the introduction of the generic will have another consequence that will be crucial for tens of thousands of people suffering from two other conditions treatable with apixaban: deep vein thrombosis (DVT) and pulmonary embolism (PE). “These indications are not covered by the public health system, so patients have to pay the full 81.96 euros. At the counter, you see that for many of them, paying this amount each month is a significant burden, while others simply cannot afford it,” explains Julio Carbó, a pharmacy owner in Barcelona.
Sources from the Ministry of Health have confirmed that the price decrease of apixaban will free up economic resources to finance these two indications shortly. This will directly benefit patients —around 45,000, according to sector sources— who were previously paying the full 82 euros per month for treatment (now reduced to 4.2 euros) and an undetermined number of people for whom this price was unaffordable. “These are good news. The role of generics is precisely to provide quality and effective medications at a lower cost once innovation has been rewarded. When they reach pharmacies, there is a price decrease that makes them more affordable. In this case, it becomes particularly evident with the prevention and treatment of deep vein thrombosis and pulmonary embolism, which will benefit many patients,” highlights Manuel Anguita, spokesperson for the Spanish Society of Cardiology (SEC).
The legal dispute between Teva and BMS has had a significant impact on the sector in recent weeks due to the magnitude of the economic figures —apixaban is the third highest-selling drug in pharmacies generating costs for the public health system— and the precautionary measures decreed by the Commercial Court 13 of Madrid, which prevented the generic from entering the market. César Hernández, Director General of Pharmacy at the Ministry of Health, expressed his dissatisfaction two weeks ago to EL PAÍS about the harm caused to the public health system by such measures, as they ultimately make the public health system bear costs resulting from disputes between private companies. The legal dispute did not concern differences in the drug’s patent duration, which is valid until 2026, but because Teva believes this protection should be revoked due to the lack of two basic elements in its processing: “sufficient description” and “inventive activity”. This implies, according to the company, that the monopoly granted by the patent is not justified.
Teva turned to a court in Barcelona and in February won the case in its favor. BMS then appealed to the Provincial Court of Barcelona, but also requested the Madrid court to impose precautionary measures. These caught Teva with hundreds of thousands of boxes ready to be distributed to pharmacies and also surprised the sector because a judge in Madrid suspended the application of a sentence issued by a Barcelona court. The Supreme Court has now addressed Teva’s claims against the precautionary measures. In a decision issued on April 24 by the Civil Chamber, the court “declares that the court number 13 of Madrid lacks functional competence” to impose the precautionary measures preventing the arrival of the apixaban generic to pharmacies. Rafael Borràs, Teva’s Director of Corporate Affairs and Market Access, sees the Supreme Court’s decision positively, citing the benefits for patients and the National Health System. A BMS spokesperson stated that the company is evaluating the process and cannot comment further at this time due to ongoing legal proceedings.