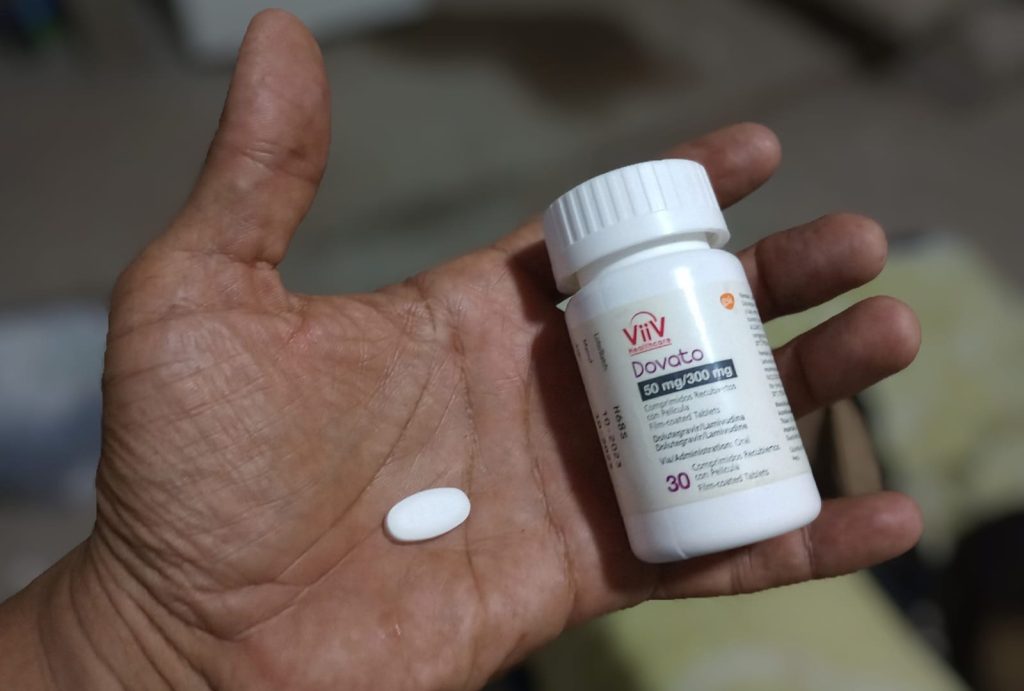
The introduction of dolutegravir into more health programs in Colombia is contingent upon how many generic doses the government purchases and how quickly they are able to do so. Typically, the registration process for generics takes at least six months, but there is urgency to secure doses while the drug is still under patent. ViiV Healthcare’s patent for dolutegravir in Colombia is set to expire in 2026, so timely action is crucial. However, concerns have been raised about Colombia’s ability to efficiently purchase and distribute the drug, citing previous issues with shortages of other medications due to delays in negotiations and acquisition.
Nestor Alvarez Lara, president of the advocacy group High Cost Patients, emphasized the need for a well-planned rollout of generic dolutegravir, urging the government to buy in large quantities to avoid potential shortages. He highlighted the importance of ensuring a continuous supply to prevent interruptions in treatment for patients. The Colombian Ministry of Health and Social Protection stated that they are finalizing negotiations to purchase a continuous supply of dolutegravir through the Pan American Health Organization, with an expected availability by the end of 2024. This plan includes purchasing over 800,000 bottles to provide a year’s worth of treatment for approximately 67,000 people.
One individual, Villan, shared his experience of switching to dolutegravir and the positive impact it had on his mental health and overall well-being. He described how the medication not only transformed his mental state but also helped him reconnect with his husband. Villan has since assisted friends in accessing dolutegravir when they faced issues with their health insurance coverage, highlighting the importance of consistent treatment for effectiveness. He expressed hope that the new license for generic dolutegravir will expand access and provide certainty for those relying on the medication.
The availability of generic dolutegravir is essential for individuals living with HIV in Colombia, as it offers a more affordable and accessible option for treatment. Given the potential challenges in the procurement process, there is a need for a coordinated and efficient approach to ensure a continuous supply of the medication. Advocates and stakeholders emphasize the importance of proper planning and foresight to avoid any delays or shortages that could impact patients’ health and well-being.
As Colombia works towards finalizing negotiations for the purchase of generic dolutegravir, there is optimism that this will enhance access to the medication for those in need. The government’s commitment to securing a continuous supply through international organizations is a positive step towards ensuring sustainability in treatment provision. Patients like Villan, whose lives have been positively impacted by dolutegravir, serve as a testament to the importance of continuous, effective treatment in managing HIV and improving overall quality of life.
In conclusion, the availability and procurement of generic dolutegravir in Colombia are crucial for expanding access to effective HIV treatment. While there are concerns about the government’s ability to efficiently purchase and distribute the medication, steps are being taken to secure a continuous supply through international partnerships. Patients, advocates, and stakeholders emphasize the necessity of proper planning and coordination to prevent interruptions in treatment and ensure the well-being of those living with HIV. Ultimately, the introduction of generic dolutegravir has the potential to improve the lives of many individuals in Colombia and contribute to better health outcomes for the community.