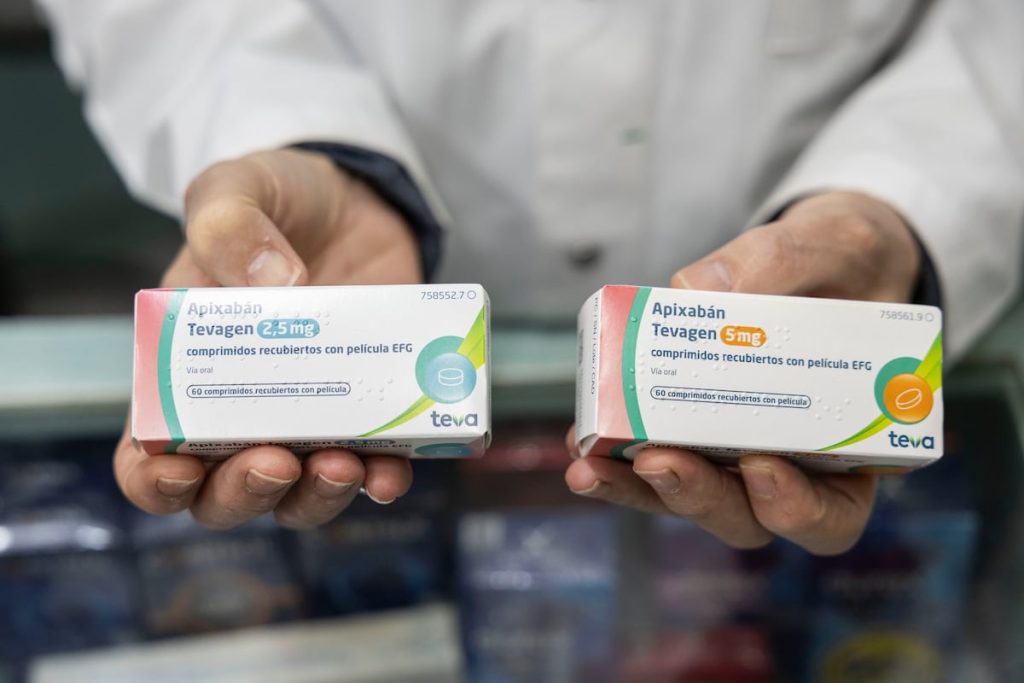
The Supreme Court last week lifted the precautionary measures that prevented the commercialization of the generic version of Apixaban, a blood thinner used by around 350,000 people in Spain. The patent holder of the medication went to a commercial court in Madrid to stop a decision made by another civil court in Barcelona. Faced with a jurisdictional conflict, the Supreme Court concluded that the first decision was the one that mattered and that the Madrid court was incompetent to intervene in the case. It is estimated that the delay has cost the Spanish public health system 23.2 million euros. The autonomous communities have been financing brand-name medications for over a month while the generics waited in a warehouse for judicial approval to be sent to pharmacies. The same afternoon the precautionary measures were lifted, the first boxes reached the hands of pharmacists.
The protection of patents is the soul of the pharmaceutical industry. It is what keeps continuous innovation alive: thousands of people in laboratories around the world are seeking a new patent that surpasses the previous one and keeps humanity at the fragile forefront of the fight against disease, against hundreds of different diseases. But that protection must be carried out within a fair and transparent legal framework. It does not seem reasonable that, in the face of a defeat in court, a jurisdictional conflict is forced that has to end up in the hands of the Supreme Court to block the distribution of a generic medication. Especially when the main victim of that conflict is the citizen: whether they do not have the medication covered by Social Security and have to pay extra, or the taxpayer who has to bear the additional costs of the brand-name compound.
Situations like this highlight the importance of generics in controlling the growing pharmaceutical expenditure of Spain, a role that must be on par with other European countries. In Spain, where cases of medicine shortage in pharmacies have tripled in just two years, the increased presence of generics in pharmacies ensures supply alternatives. In recent years, there has been a focus on the need to boost the market share of generics and several measures have been considered, although nothing has yet been published in the Official State Gazette. All – industry, administration, and the public – benefit from a balanced innovation system that justifies the development costs of new medications and at the same time allows already amortized patents to have a broader market. Spain is moving in that direction and the pharmaceutical sector should not hinder this progress.