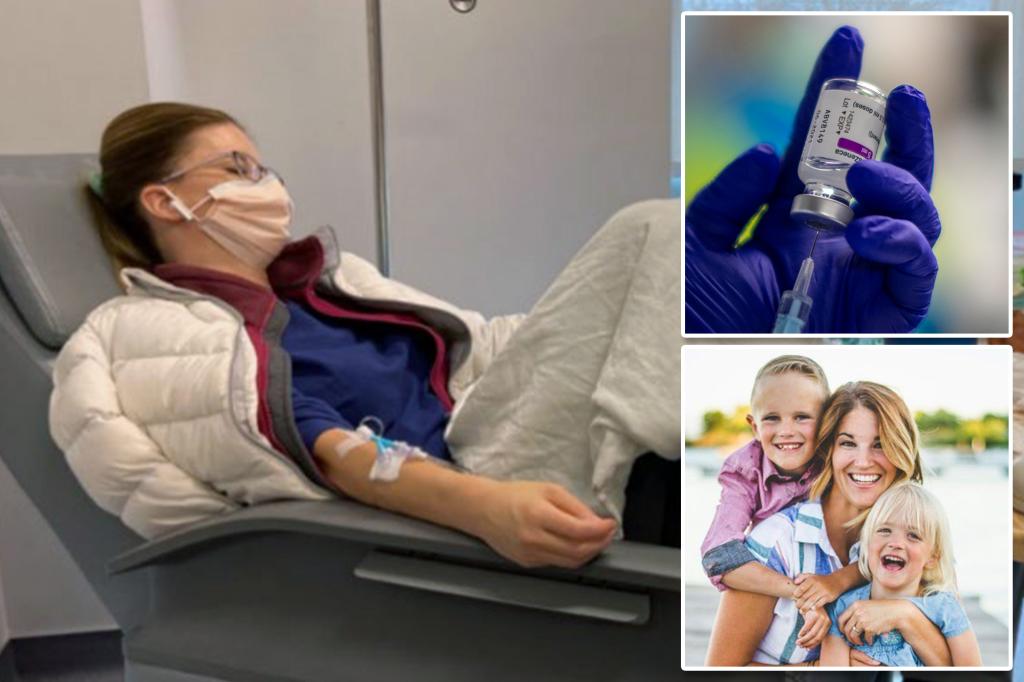
Brianne Dressen, a 42-year-old mother from Utah, participated in a clinical trial for AstraZeneca’s COVID-19 vaccine and ended up suffering severe side effects that left her permanently disabled. She alleges that the vaccine trial led to a neurological condition called paresthesia, which caused her extreme discomfort and medical complications. Dressen claims that her medical bills have become exorbitant, but AstraZeneca has failed to adequately compensate her as promised in the consent form she signed before joining the trial.
Following her injection with the vaccine, Dressen experienced arm tingles, blurred vision, headaches, ringing ears, and vomiting, with symptoms eventually spreading to her legs. Despite multiple hospitalizations and a diagnosis of post-vaccine neuropathy by neurologists, AstraZeneca reportedly only offered her a one-time payment of $1,243.30 to cover her medical expenses. Dressen’s lawsuit against the drug manufacturer alleges that they breached their contract by not providing sufficient compensation for her ongoing medical needs and lost wages resulting from her disability.
While some of Dressen’s symptoms have improved, she maintains that she is still disabled and struggling to care for her two children as she once did. She feels betrayed by AstraZeneca for not living up to their promise of covering research-related medical costs and feels abandoned by the company she trusted during the clinical trial. Dressen’s case is one of many, as more than 50 individuals have joined a class-action lawsuit against AstraZeneca in the UK, alleging similar experiences of adverse effects from the vaccine.
Despite the lawsuits and claims of adverse effects, AstraZeneca stands by the safety and efficacy of their vaccine, stating that it has been shown to have an acceptable safety profile in both clinical trials and real-world data. Regulators globally have consistently supported the benefits of vaccination with the AstraZeneca-Oxford vaccine outweighing the potential risks of rare side effects. The vaccine, while not approved for use in the US, has been credited with saving millions of lives worldwide during the COVID-19 pandemic, according to the University of Oxford.
Dressen’s story represents the challenges faced by individuals who participate in clinical trials and suffer unexpected consequences. The emotional and financial toll on patients like Dressen, who believed they were contributing to public health through vaccine research, highlights the importance of transparency, accountability, and adequate support for participants in clinical trials. As the debate over vaccine safety and accountability continues, cases like Dressen’s serve as a reminder of the complexities and risks involved in medical research and the need for a fair and just response to those who experience harm. Ultimately, addressing these issues will be crucial in maintaining trust in the scientific community and ensuring the safety and well-being of all participants in clinical trials.